

Your vaccination provider may recommend that you get your next COVID-19 vaccine in the opposite arm if possible. These rashes are also known as “COVID arm.” Tell your vaccination provider that you experienced a rash or “COVID arm” after your shot. These rashes can start a few days to more than a week after your shot and are sometimes quite large. Your vaccination provider may recommend that you get your next COVID-19 vaccine in the opposite arm, if possible. This applies to second, additional, or booster shots. If you had a red, itchy, swollen, or painful rash where you got a COVID-19 shot, you should still get another shot at the scheduled date and time. If You Had a Rash on the Arm where You Got a COVID-19 Shot Your doctor may refer you to an allergy and immunology specialist for additional care or advice. If you had an immediate allergic reaction (a reaction that started within 4 hours of getting vaccinated) to a COVID-19 vaccine, but the reaction was not considered severe by a medical professional, you likely can receive another dose of the same vaccine under certain conditions. If You Had a Non-severe Allergic Reaction to a COVID-19 Vaccine However, your doctor may refer you to an allergy and immunology specialist for additional care or advice. If you have had an immediate allergic reaction (a reaction that started within 4 hours) to any vaccine other than a COVID-19 vaccine or any injectable therapy, you may still be able to get a COVID-19 vaccine. If You Have Had an Immediate Allergic Reaction to Other Vaccines or Injectables Learn about getting a different type of COVID-19 vaccine after an allergic reaction. However, if you had a severe allergic reaction after a dose of an mRNA COVID-19 vaccine or if you have had a severe allergic reaction to any ingredient in an mRNA COVID-19 vaccine, you may be able to get the J&J/Janssen COVID-19 vaccine.

If you had a severe allergic reaction after receiving a particular type of COVID-19 vaccine (either mRNA, protein subunit, or viral vector), you should not get another dose of that type of vaccine.ĬDC recommends that people getting a booster get an mRNA COVID-19 vaccine (Pfizer-BioNTech or Moderna).Novavax COVID-19 vaccine is a protein subunit vaccine.Johnson & Johnson’s/Janssen (J&J/Janssen) COVID-19 vaccine is a viral vector vaccine.The Pfizer-BioNTech and Moderna COVID-19 vaccines are messenger RNA vaccines, also called mRNA vaccines.If You Had a Severe Allergic Reaction to a COVID-19 Vaccine
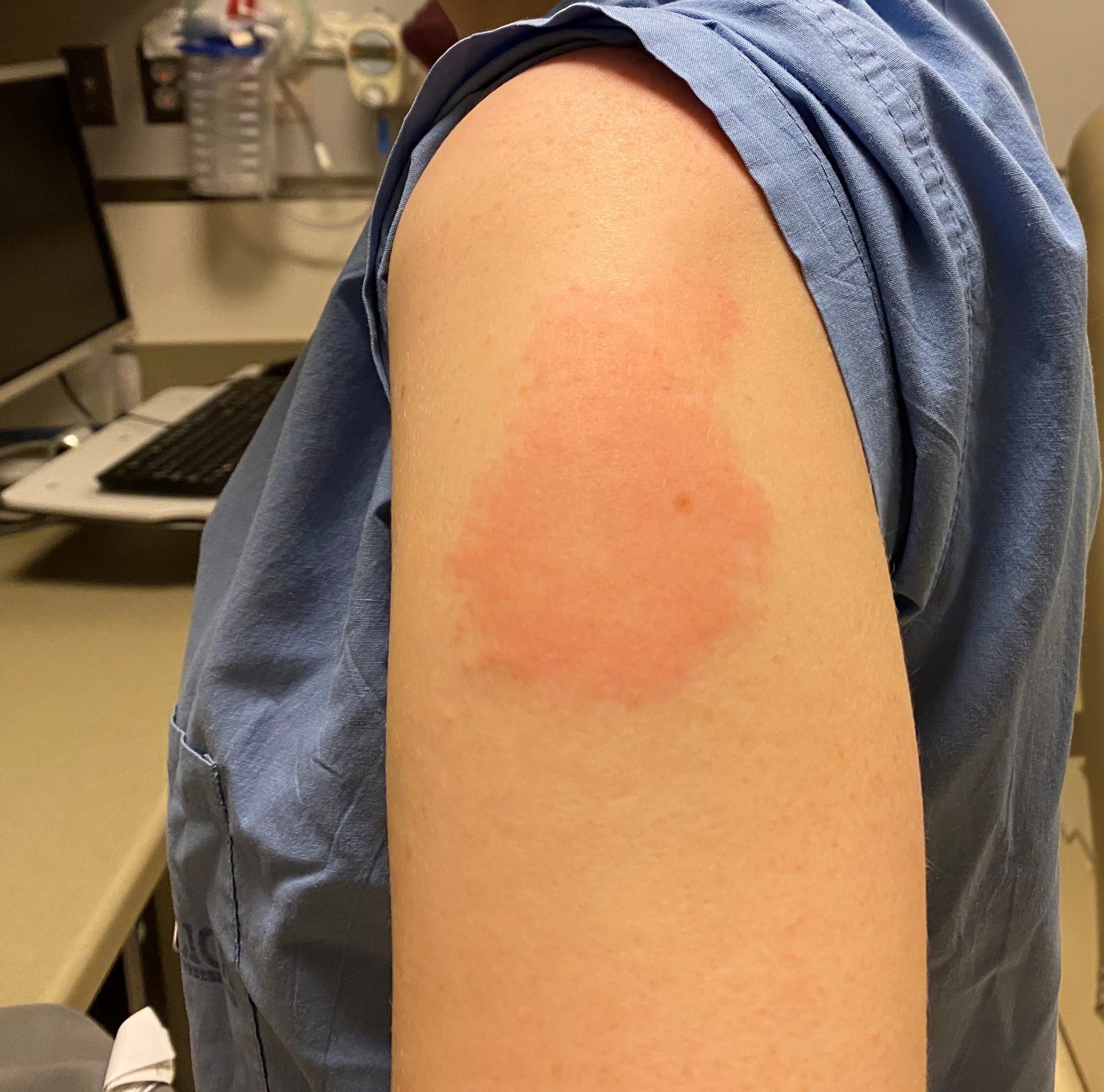
a generalized rash or hives, which may include mucus membranes.Nearly a million doses from the lot have already been distributed to about 1,700 vaccination sites in 37 states, Moderna said. health regulators to understand the cases and whether pausing the use of the lot was warranted. Moderna said it was working closely with U.S. The vaccine maker said it was unaware of comparable cases of adverse events from other vaccination centers which may have administered vaccines from the same lot or from other lots of its vaccine.Ī total of 307,300 doses from the lot remain in storage, Moderna said, of the total 1,272,200 doses that were produced in the batch. Newsletter sign-up: Get The COVID-19 Brief sent to your inbox.Fewer than 10 individuals required medical attention over the span of 24 hours," the epidemiologist said in a statement "A higher-than-usual number of possible allergic reactions were reported with a specific lot of Moderna vaccine administered at one community vaccination clinic. 41L20A due to possible allergic reactions that are under investigation. The company's comments come after California's top epidemiologist on Sunday issued a statement recommending providers pause vaccination from lot no. Moderna Inc said on Tuesday it had received a report from California's health department that several people at a center in San Diego were treated for possible allergic reactions to its COVID-19 vaccine from a particular batch.


 0 kommentar(er)
0 kommentar(er)
